Recce Pharmaceuticals Reports Positive Preclinical Data of RECCE® 327 in Lung Infection Pilot Study
- RECCE® 327 (R327) demonstrated >99% log reduction (>2.5 log reduction) in Mycobacterium abscessus lung infections using a nebulizer delivery method
- R327 is being evaluated for potential use to treat ventilator-associated pneumonia (VAP) and hospital-acquired pneumonia (HAP)
SYDNEY, Australia, May 13, 2024 (GLOBE NEWSWIRE) -- Recce Pharmaceuticals Ltd. (ASX: RCE, FSE: R9Q) (the Company), the Company developing a new class of synthetic anti-infectives, today announced positive results from a preclinical pilot study evaluating nebulized RECCE® 327 (R327) for the treatment of lung infections in a mouse model. The study was conducted at the company’s Anti-Infective Research (AIR) unit within Murdoch Children’s Research Institute.
“These results represent a significant milestone in the development of nebulized treatments for lung infections,” said James Graham, CEO of Recce Pharmaceuticals. “The ability of R327 to significantly decrease bacterial infections in the lungs without adverse effects on the host is a testament to its potential as a safe and effective treatment option.
Sohinee Sarkar, Ph.D., lead researcher at Recce’s AIR unit, added, “The results are very promising and pave the way for future clinical applications. This could be particularly transformative for patients suffering from VAP and HAP, conditions that significantly increase morbidity and mortality rates in intensive care units.”
The pilot study demonstrated a significant reduction in Mycobacterium abscessus (M. abscessus) colonization in both lungs of mice treated with nebulized R327. Notably, the mice maintained a stable body weight throughout the study period, indicating the treatment’s safety and tolerability. This pilot study represents an important step toward exploring new methods of administration across a broad range of therapeutic indications.
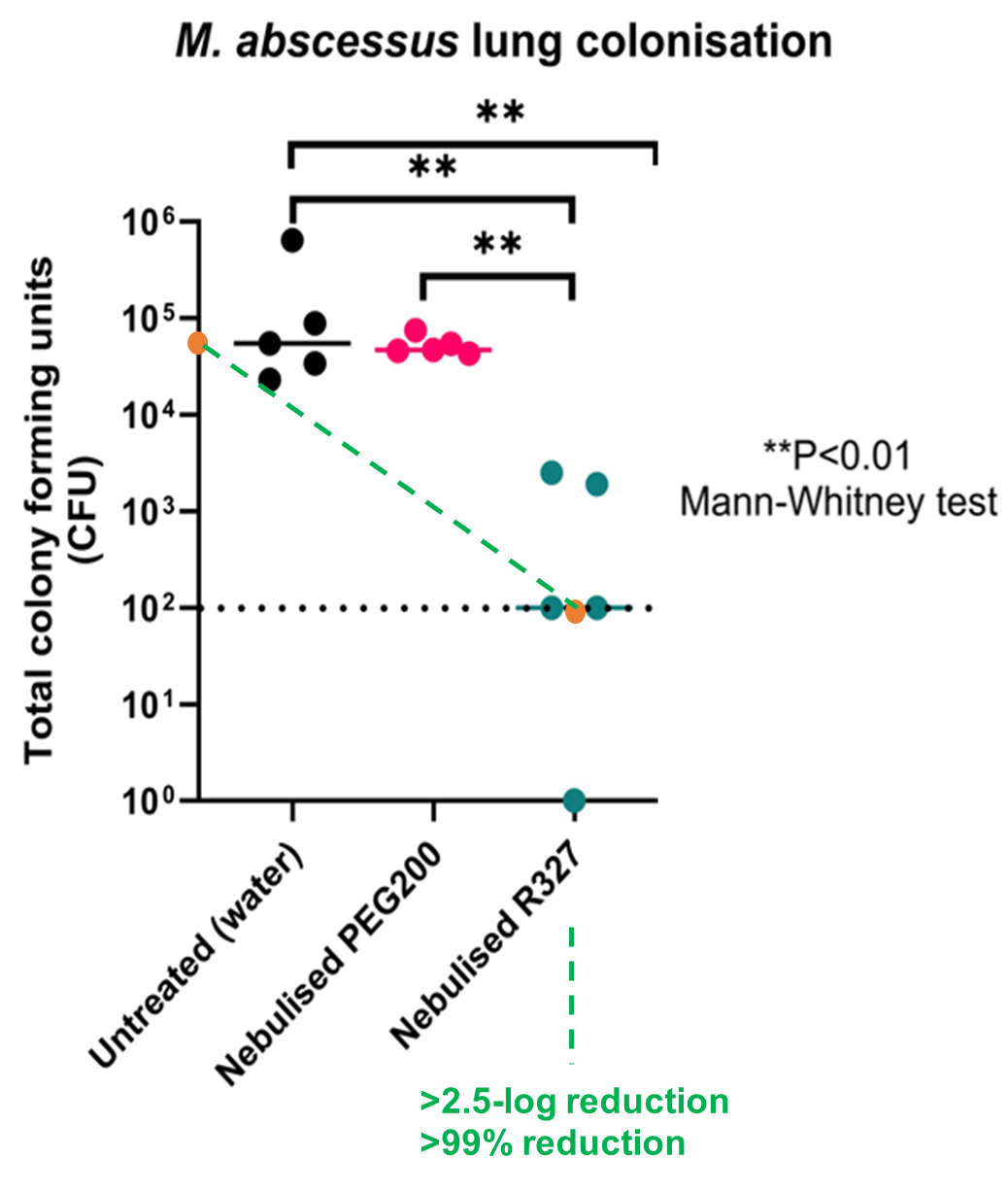
The data builds on past preclinical studies where R327 demonstrated a dose-dependent killing of intracellular M. abscessus with no toxicity observed against treated human macrophages or in intranasally infected mice. Furthermore, R327 was shown to be superior to the positive control clarithromycin (CLA), a current treatment.1
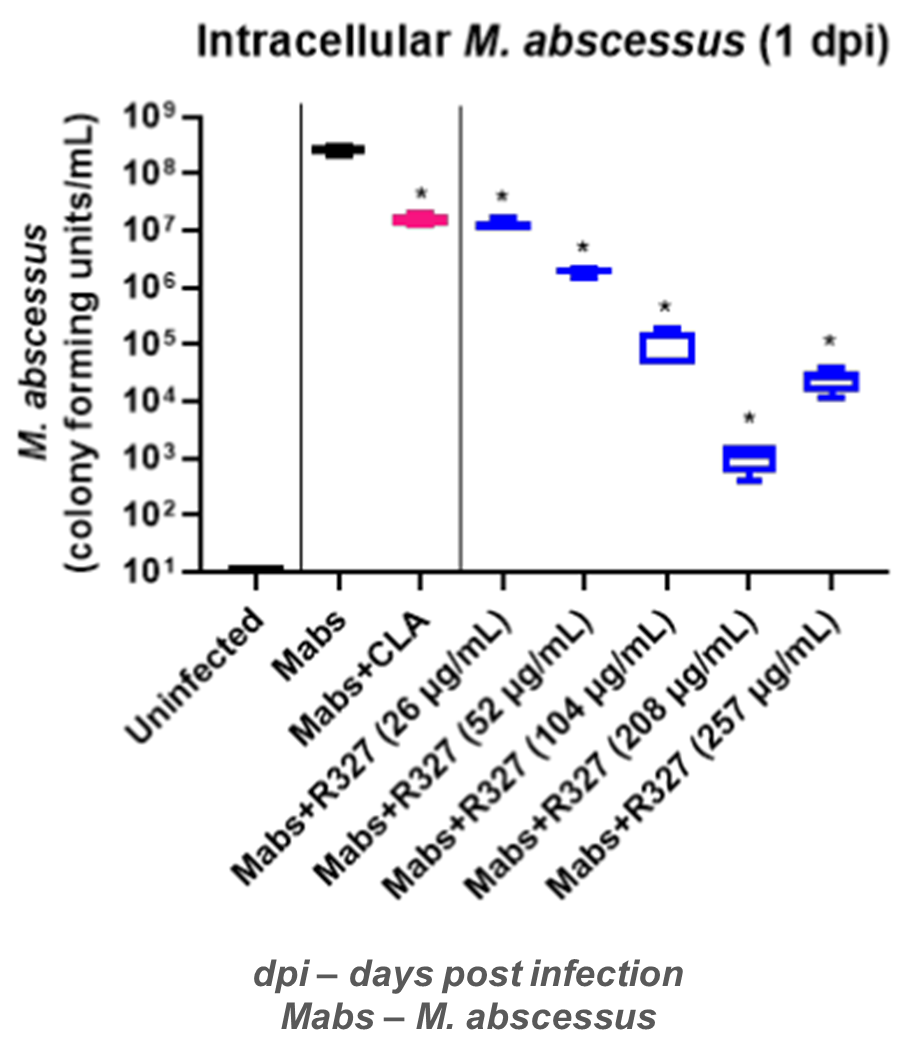
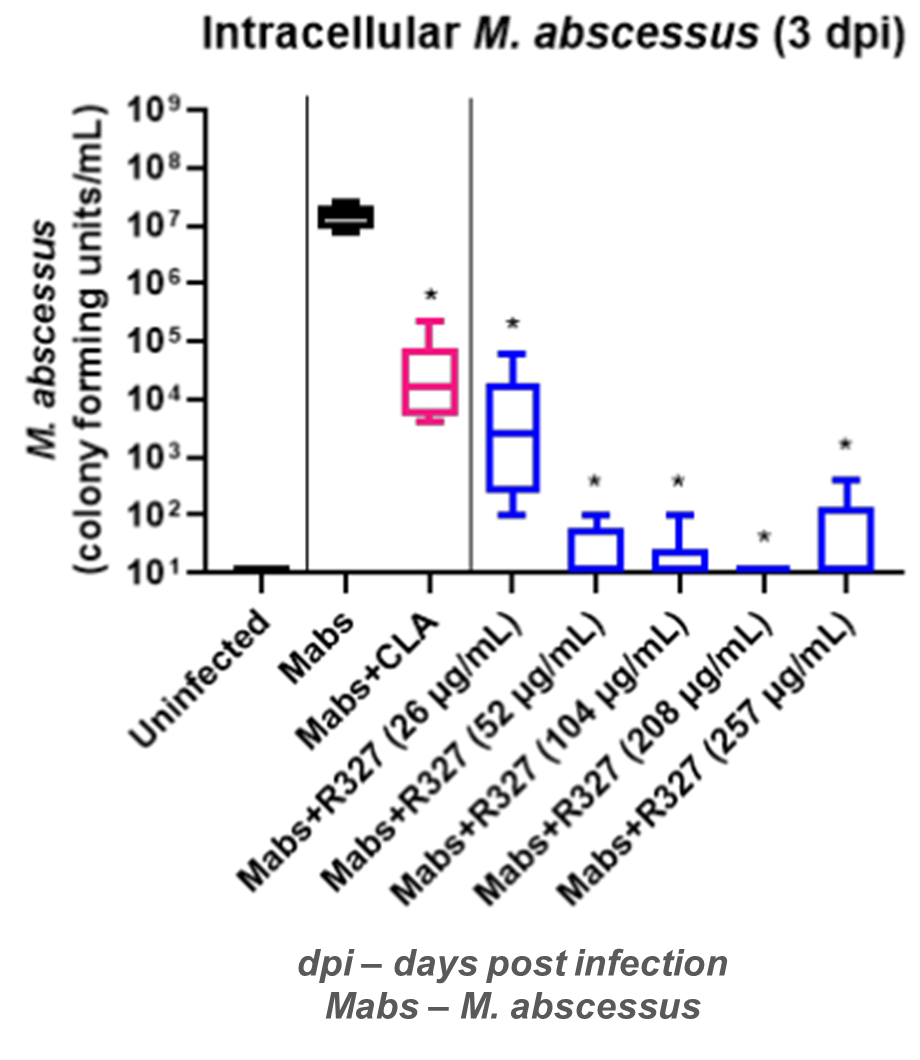
Infections due to M. abscessus are a major cause of mortality and morbidity in cystic fibrosis (CF) patients.2 Current treatment guidelines recommend a prolonged and intense combination therapy consisting of several antibiotic agents with significant adverse effects.3
Ventilator-associated pneumonia (VAP) is a major concern in healthcare settings, occurring in 9-27% of mechanically ventilated patients.4 Given the high incidence and severity of VAP, R327’s results suggest it could fill a critical gap in the treatment of VAP or similar infections. Hospital-acquired pneumonia (HAP), one of the most common nosocomial infections, is associated with significant clinical and economic burdens, such as long-term hospitalization, high medical costs, and increased morbidity and mortality.5
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q) is developing a New Class of Synthetic Anti-Infectives designed to address the urgent global health problems of antibiotic-resistant superbugs and emerging viral pathogens.
Recce’s anti-infective pipeline includes three patented, broad-spectrum, synthetic polymer anti-infectives: RECCE® 327 (R327) as an intravenous and topical therapy that is being developed for the treatment of serious and potentially life-threatening infections due to Gram-positive and Gram-negative bacteria, including their superbug forms; RECCE® 435 (R435) as an orally administered therapy for bacterial infections; and RECCE® 529 (R529) for viral infections. Through their multi-layered mechanisms of action, Recce’s anti-infectives have the potential to overcome the processes utilised by bacteria and viruses to overcome resistance – a current challenge facing existing antibiotics.
The World Health Organization (WHO) added R327, R435, and R529 to its list of antibacterial products in clinical development for priority pathogens, recognising Recce’s efforts to combat antimicrobial resistance. The FDA granted R327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act, providing Fast Track Designation and 10 years of market exclusivity post approval. R327 is also included on The Pew Charitable Trusts’ Global New Antibiotics in Development Pipeline as the sole synthetic polymer and sepsis drug candidate in development.
Recce wholly owns its automated manufacturing, supporting current clinical trials. Recce’s anti-infective pipeline aims to address synergistic, unmet medical needs by leveraging its unique technologies.
Corporate Contact
James Graham
Recce Pharmaceuticals Ltd
+61 (02) 9256 2571
James.graham@recce.com.au
Media & Investor Relations (AU)
Andrew Geddes
CityPR
+61 (02) 9267 4511
ageddes@citypublicrelations.com.au
Media (USA)
Michael Fitzhugh
LifeSci Communications
mfitzhugh@lifescicomms.com
Investor Relations (USA & EU)
Guillame van Renterghem
LifeSci Advisors
gvanrenterghem@lifesciadvisors.com
1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5192163/
2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9431180/
3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9431180/
4 https://ccforum.biomedcentral.com/articles/10.1186/cc13775#:~:text=VAP%20is%20estimated%20to%20occur,4%5D%2C%20%5B5%5D
5 https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-021-01816-9
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/5a2526c2-9405-4549-950f-f7436c907a7c
https://www.globenewswire.com/NewsRoom/AttachmentNg/8617b989-ccd3-4ddf-bc1a-5f9d36ad5108
https://www.globenewswire.com/NewsRoom/AttachmentNg/b765c93c-9fb9-47ec-ad7a-0c0f93c22628